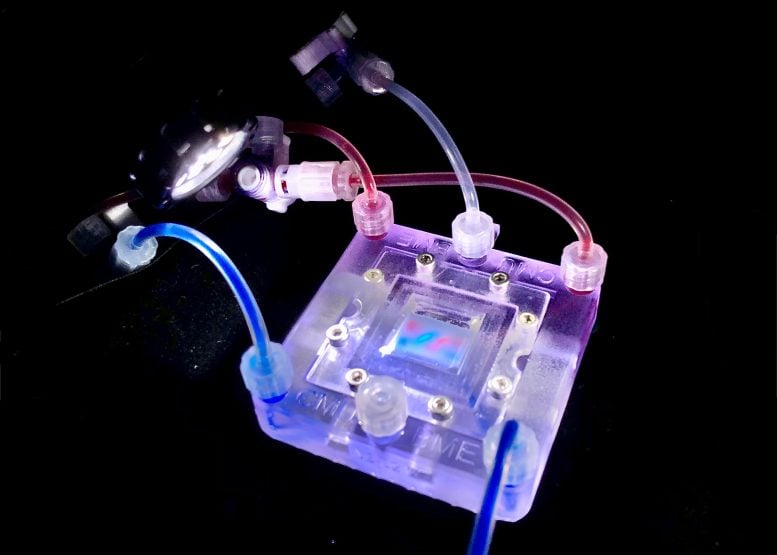
Researchers at the University of Pittsburgh have created a groundbreaking tissue engineering platform using 3D-printed collagen scaffolds called CHIPS.
By mimicking natural cellular environments, they enable cells to grow, interact, and form functional tissues — a major step beyond traditional silicone-based microfluidic models. The platform not only models diseases like diabetes but could also replace animal testing in the future. Plus, their designs are freely available to fuel broader scientific innovation.
3D Bioprinting: Turning Science Fiction Into Science Reality
Creating organic tissue models that behave like living organs might sound like science fiction, but engineers at the University of Pittsburgh are turning it into reality.
At the heart of their breakthrough is a powerful concept: given the right environment, cells naturally know how to organize and function. The key is designing scaffolds that mimic the body’s natural structures, providing the cues cells need to grow, interact, and form tissues.
Daniel Shiwarski, an assistant professor of bioengineering at the Swanson School of Engineering and a faculty member at the School of Medicine’s Vascular Medicine Institute, developed a new type of scaffold called “CHIPS” — collagen-based, high-resolution, internally perfusable structures. These CHIPS integrate with a vascular and perfusion organ-on-a-chip reactor, creating a complete tissue engineering platform that closely simulates a real cellular environment.
In collaboration with Adam Feinberg, a professor of biomedical engineering at Carnegie Mellon University, Shiwarski’s team published their findings in the April edition of <span class="glossaryLink" aria-describedby="tt" data-cmtooltip="
” data-gt-translate-attributes=”[{"attribute":"data-cmtooltip", "format":"html"}]” tabindex=”0″ role=”link”>Science Advances, where the study, titled “3D Bioprinting of collagen-based high resolution internally perfusable scaffolds for engineering fully biologic tissue systems,” was featured as the cover story.
Revolutionizing Disease Modeling With Natural Materials
Shiwarski’s research leverages additive manufacturing and tissue engineering to create functional replacement tissues and model diseases like diabetes and hypertension. A popular method of studying these diseases in vitro, microfluidic modeling, uses tiny channels in a small chip to simulate blood vessel or cellular behavior. These models are typically made from silicone, and while useful, their synthetic nature has restricted scientists from utilizing these models to their full potential—until now.
“Microfluidic devices help us study cell behavior, but they’re inherently limited,” Shiwarski said. “Our collagen-based scaffolds change that. Since cells naturally thrive in collagen, we can print not only the structural network but also embed cells directly into that environment, allowing them to grow, interact, and form tissues.”
Real Tissue Formation: Cells Thriving in Collagen Networks
Unlike traditionally synthetic microfluidic devices, these printed scaffolds were built entirely from collagen, allowing cells to interact with the model itself by growing and self-organizing into functional tissues within it. The team demonstrated this by combining the collagen with vascular and pancreatic cells, prompting <span class="glossaryLink" aria-describedby="tt" data-cmtooltip="
” data-gt-translate-attributes=”[{"attribute":"data-cmtooltip", "format":"html"}]” tabindex=”0″ role=”link”>insulin secretion in response to glucose, which mirrored natural physiological function.
To support the growth and development of cellularized collagen scaffolds, the team engineered a custom perfusion bioreactor system, VAPOR.
”This platform is unique as it securely connects the soft collagen-based tissue scaffolds to the VAPOR fluidic system by snapping the CHIPS into place around like Lego blocks,” said Andrew Hudson, co-founder of FluidForm Bio and publication co-author.
Breaking New Ground With 3D Vascular Networks
Additionally, while traditional microfluidic devices are limited in design to flat or sequentially layered patterns, the team also demonstrated the ability to create non-planar 3D networks in soft, organic material by printing helical vascular networks modeled after <span class="glossaryLink" aria-describedby="tt" data-cmtooltip="
” data-gt-translate-attributes=”[{"attribute":"data-cmtooltip", "format":"html"}]” tabindex=”0″ role=”link”>DNA structure.
“We’re taking everything that works well in microfluidics—like controlling fluid flow and setting up vascular networks—and combining it with natural biomaterials and the innate programming of cells,” Shiwarski said. “If we place cells in an environment that mimics their natural surroundings, they know exactly what to do. We’re recreating the right environment for them and letting the cells do their job, allowing them to adapt, evolve, and build functional tissue over time.”
Future Frontiers: Modeling Disease Without Animal Testing
Shiwarski is also committed to open science with his team’s work; all models and designs from the project are freely available on his lab’s website. Looking ahead, Shiwarski’s team aims to use this platform to study vascular diseases such as hypertension and fibrosis, modeling how these conditions affect tissue development and function. The ultimate goal is to eventually replace animal models with more accurate, human-based systems.
“This new approach lets us bridge the gap between simplified 2D models and animal studies,” Shiwarski said. “Now that we’ve established this functional tissue environment, one of our next big goals is to study how vascular networks form alongside the development of underlying tissues—and how these processes are affected by human-specific disease variants. We can use this as a way to study complicated diseases and understand the basic biology behind them, and then we can get further insight into clinical therapies.”
Reference: “3D bioprinting of collagen-based high-resolution internally perfusable scaffolds for engineering fully biologic tissue systems” by Daniel J. Shiwarski, Andrew R. Hudson, Joshua W. Tashman, Ezgi Bakirci, Samuel Moss, Brian D. Coffin and Adam W. Feinberg, 23 April 2025, Science Advances.
DOI: 10.1126/sciadv.adu5905
