
<span class="glossaryLink" aria-describedby="tt" data-cmtooltip="
” data-gt-translate-attributes=”[{"attribute":"data-cmtooltip", "format":"html"}]” tabindex=”0″ role=”link”>MIT researchers have developed a revolutionary sodium–air fuel cell that could replace heavy lithium-ion batteries in aviation, rail, and marine transport.
Using liquid sodium and ambient air, this system offers triple the energy density of current EV batteries — potentially enabling electric aircraft. The cell emits no carbon dioxide and even captures CO2 from the air, producing baking soda as a byproduct.
Fuel Cell Breakthrough for Transport Electrification
Traditional batteries are starting to hit their limit when it comes to how much energy they can store for their weight. That’s a big roadblock for the future of electric transportation, especially for energy-hungry machines like airplanes, trains, and ships. Now, a team of researchers at MIT and their collaborators may have found a way around it — and it’s exciting.
Instead of a battery, their new design is a fuel cell. Like a battery, it generates electricity through chemical reactions, but there’s a major advantage: it can be refueled quickly rather than slowly recharged. This particular fuel cell uses liquid sodium metal, a low-cost and widely available material, as its energy source. The other ingredient? Ordinary air. A solid ceramic layer between them helps sodium ions move through, while a special electrode on the air side sparks a reaction that produces electricity.
Three Times the Energy Density of Lithium-Ion
When the team tested a prototype, the results were striking. The sodium-air fuel cell stored more than three times as much energy per kilogram as the lithium-ion batteries used in today’s electric vehicles. That’s a massive leap forward. The findings, published on May 27 in the journal Joule, come from MIT doctoral students Karen Sugano, Sunil Mair, Saahir Ganti-Agrawal, Professor Yet-Ming Chiang, and colleagues.

High Energy Density Could Enable Electric Flight
“We expect people to think that this is a totally crazy idea,” says Chiang, who is the Kyocera Professor of Ceramics. “If they didn’t, I’d be a bit disappointed because if people don’t think something is totally crazy at first, it probably isn’t going to be that revolutionary.”
And this technology does appear to have the potential to be quite revolutionary, he suggests. In particular, for aviation, where weight is especially crucial, such an improvement in energy density could be the breakthrough that finally makes electrically powered flight practical at significant scale.
“The threshold that you really need for realistic electric aviation is about 1,000 watt-hours per kilogram,” Chiang says. Today’s electric vehicle lithium-ion batteries top out at about 300 watt-hours per kilogram — nowhere near what’s needed. Even at 1,000 watt-hours per kilogram, he says, that wouldn’t be enough to enable transcontinental or trans-Atlantic flights.
That’s still beyond reach for any known battery chemistry, but Chiang says that getting to 1,000 watts per kilogram would be an enabling technology for regional electric aviation, which accounts for about 80 percent of domestic flights and 30 percent of the emissions from aviation.
The technology could be an enabler for other sectors as well, including marine and rail transportation. “They all require very high energy density, and they all require low cost,” he says. “And that’s what attracted us to sodium metal.”
Beyond Batteries: Metal-Air Fuel Cell Advantages
A great deal of research has gone into developing lithium-air or sodium-air batteries over the last three decades, but it has been hard to make them fully rechargeable. “People have been aware of the energy density you could get with metal-air batteries for a very long time, and it’s been hugely attractive, but it’s just never been realized in practice,” Chiang says.
By using the same basic electrochemical concept, only making it a fuel cell instead of a battery, the researchers were able to get the advantages of the high energy density in a practical form. Unlike a battery, whose materials are assembled once and sealed in a container, with a fuel cell the energy-carrying materials go in and out.

Prototypes Showcase Feasible Cell Designs
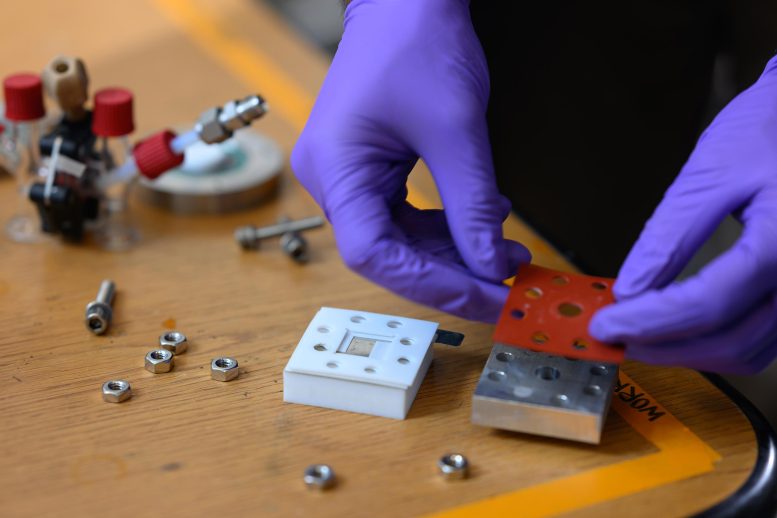
The team produced two different versions of a lab-scale prototype of the system. In one, called an H cell, two vertical glass tubes are connected by a tube across the middle, which contains a solid ceramic electrolyte material and a porous air electrode. Liquid sodium metal fills the tube on one side, and air flows through the other, providing the oxygen for the electrochemical reaction at the center, which ends up gradually consuming the sodium fuel. The other prototype uses a horizontal design, with a tray of the electrolyte material holding the liquid sodium fuel. The porous air electrode, which facilitates the reaction, is affixed to the bottom of the tray.
Tests using an air stream with a carefully controlled humidity level produced a level of more than 1,500 watt-hours per kilogram at the level of an individual “stack,” which would translate to over 1,000 watt-hours at the full system level, Chiang says.
Zero Carbon Emissions — And Carbon Capture
The researchers envision that to use this system in an aircraft, fuel packs containing stacks of cells, like racks of food trays in a cafeteria, would be inserted into the fuel cells; the sodium metal inside these packs gets chemically transformed as it provides the power. A stream of its chemical byproduct is given off, and in the case of aircraft this would be emitted out the back, not unlike the exhaust from a jet engine.
But there’s a very big difference: There would be no carbon dioxide emissions. Instead, the emissions, consisting of sodium oxide, would actually soak up carbon dioxide from the atmosphere. This compound would quickly combine with moisture in the air to make sodium hydroxide — a material commonly used as a drain cleaner — which readily combines with carbon dioxide to form a solid material, sodium carbonate, which in turn forms sodium bicarbonate, otherwise known as baking soda.
“There’s this natural cascade of reactions that happens when you start with sodium metal,” Chiang says. “It’s all spontaneous. We don’t have to do anything to make it happen, we just have to fly the airplane.”
As an added benefit, if the final product, the sodium bicarbonate, ends up in the ocean, it could help to de-acidify the water, countering another of the damaging effects of greenhouse gases.
Using sodium hydroxide to capture carbon dioxide has been proposed as a way of mitigating carbon emissions, but on its own, it’s not an economic solution because the compound is too expensive. “But here, it’s a byproduct,” Chiang explains, so it’s essentially free, producing environmental benefits at no cost.
Built-In Safety: Why Fuel Cells May Beat Batteries
Importantly, the new fuel cell is inherently safer than many other batteries, he says. Sodium metal is extremely reactive and must be well-protected. As with lithium batteries, sodium can spontaneously ignite if exposed to moisture. “Whenever you have a very high energy density battery, safety is always a concern, because if there’s a rupture of the membrane that separates the two reactants, you can have a runaway reaction,” Chiang says. But in this fuel cell, one side is just air, “which is dilute and limited. So you don’t have two concentrated reactants right next to each other. If you’re pushing for really, really high energy density, you’d rather have a fuel cell than a battery for safety reasons.”
While the device so far exists only as a small, single-cell prototype, Chiang says the system should be quite straightforward to scale up to practical sizes for commercialization. Members of the research team have already formed a company, Propel Aero, to develop the technology. The company is currently housed in MIT’s startup incubator, The Engine.

Sodium: Abundant, Cheap, and Easy to Refuel
Producing enough sodium metal to enable widespread, full-scale global implementation of this technology should be practical, since the material has been produced at a large scale before. When leaded gasoline was the norm, before it was phased out, sodium metal was used to make the tetraethyl lead used as an additive, and it was being produced in the U.S. at a capacity of 200,000 tons a year. “It reminds us that sodium metal was once produced at large scale and safely handled and distributed around the U.S.,” Chiang says.
What’s more, sodium primarily originates from sodium chloride, or salt, so it is abundant, widely distributed around the world, and easily extracted, unlike lithium and other materials used in today’s EV batteries.
Refillable Cartridges and Drone Power Proof-of-Concept
The system they envisage would use a refillable cartridge, which would be filled with liquid sodium metal and sealed. When it’s depleted, it would be returned to a refilling station and loaded with fresh sodium. Sodium melts at 98 degrees <span class="glossaryLink" aria-describedby="tt" data-cmtooltip="
” data-gt-translate-attributes=”[{"attribute":"data-cmtooltip", "format":"html"}]” tabindex=”0″ role=”link”>Celsius, just below the boiling point of water, so it is easy to heat to the melting point to refuel the cartridges.
Initially, the plan is to produce a brick-sized fuel cell that can deliver about 1,000 watt-hours of energy, enough to power a large drone, in order to prove the concept in a practical form that could be used for agriculture, for example. The team hopes to have such a demonstration ready within the next year.
Sugano, who conducted much of the experimental work as part of her doctoral thesis and will now work at the startup, says that a key insight was the importance of moisture in the process. As she tested the device with pure oxygen, and then with air, she found that the amount of humidity in the air was crucial to making the electrochemical reaction efficient. The humid air resulted in the sodium producing its discharge products in liquid rather than solid form, making it much easier for these to be removed by the flow of air through the system. “The key was that we can form this liquid discharge product and remove it easily, as opposed to the solid discharge that would form in dry conditions,” she says.
Merging Engineering Fields for Radical Gains
Ganti-Agrawal notes that the team drew from a variety of different engineering subfields. For example, there has been much research on high-temperature sodium, but none with a system with controlled humidity. “We’re pulling from fuel cell research in terms of designing our electrode, we’re pulling from older high-temperature battery research as well as some nascent sodium-air battery research, and kind of mushing it together,” which led to the “the big bump in performance,” the team has achieved, he says.
Reference: “Sodium-air fuel cell for high energy density and low-cost electric power” by Karen Sugano, Sunil Mair, Saahir Ganti-Agrawal, Alden S. Friesen, Kailash Raman, William H. Woodford, Shashank Sripad, Venkatasubramanian Viswanathan and Yet-Ming Chiang, 27 May 2025, Joule.
DOI: 10.1016/j.joule.2025.101962
The research team also included Alden Friesen, an MIT summer intern who attends Desert Mountain High School in Scottsdale, Arizona; Kailash Raman and William Woodford of Form Energy in Somerville, Massachusetts; Shashank Sripad of And Battery Aero in California, and Venkatasubramanian Viswanathan of the University of Michigan. The work was supported by ARPA-E, Breakthrough Energy Ventures, and the National Science Foundation, and used facilities at MIT.nano.
Never miss a breakthrough: Join the SciTechDaily newsletter.
